Abstract
Introduction Allogeneic hematopoietic stem cell transplant (alloHSCT) is a mainstay of treatment for hematologic malignancies such as acute leukemias and aggressive lymphomas. Conventionally, fresh donor hematopoietic progenitor cells (HPC) are used, but the COVID-19 pandemic forced many centres to switch to cryopreservation of HPC for alloHSCT. We aimed to compare the outcomes in alloHSCT patients who received cryo-HPC with a recent historical cohort of patients who received fresh-HPC at our centre.
Methods A retrospective chart review was conducted on all adult patients who received a bone marrow (BM) or peripheral blood alloHSCT in British Columbia between June 2017 and Jan 2022; cryo-HPCs were the predominant product used as of March 2020. CIBMTR criteria were used to define engraftment. Patients who died before 30 days post-alloHSCT were excluded from primary graft failure (GF) and GVHD analysis; those who died before 100 days post-alloHSCT or had primary GF were excluded from secondary GF and chronic GVHD analysis. Patients who were alive on the date of last follow-up (January 31, 2022) were censored. Statistical significance was calculated using the Chi square test for categorical variables and T-test or Mann-Whitney U test for continuous variables. Kaplan-Meier curves were plotted for OS.
Results A total of 271 alloHSCT procedures were included in the analysis, of which 134 received fresh-HPC and 137 cryo-HPC. The median age at the time of transplant was 54 years [IQR 46-64] in the fresh-HPC vs 59 [IQR 39-61] in the cryo-HPC group (p=0.02). Male recipients comprised 60% of both groups (p=1). There was no significant difference in primary diagnosis (p=0.17); AML was the most frequent indication (46% fresh-HPC vs 35% cryo-HPC), followed by ALL/LBL (20% vs 23%). Most patients were in CR1 (63% vs 53%, p=0.01).
The median CD34+ cell count infused was 7x106/kg in the fresh and 6x106/kg in the cryo-HPC group (p=0.08). For the cryo-HPC, the median time from cell collection to cryopreservation was 33 hours [IQR 23, 55], with a median cell viability of 96% [IQR 95, 98].
Matched unrelated donor was the most common donor type, which comprised 75% alloHSCTs in the fresh-HPC and 54% in the cryo-HPC group (p<0.01). Bone marrow source was used for 8% and 5% of alloHSCTs in the fresh-HPC and cryo-HPC groups, respectively (p=0.10). Myeloablative condition was used in 78% fresh-HPC vs 72% cryo-HPC (p=0.31) and busulfan/fludarabine was the most common regimen (47% vs 58%, p=0.01). Most patients received GVHD prophylaxis with cyclosporine and methotrexate ± Thymoglobulin (96% in fresh HPC, 79% in cryo-HPC, p<0.01)
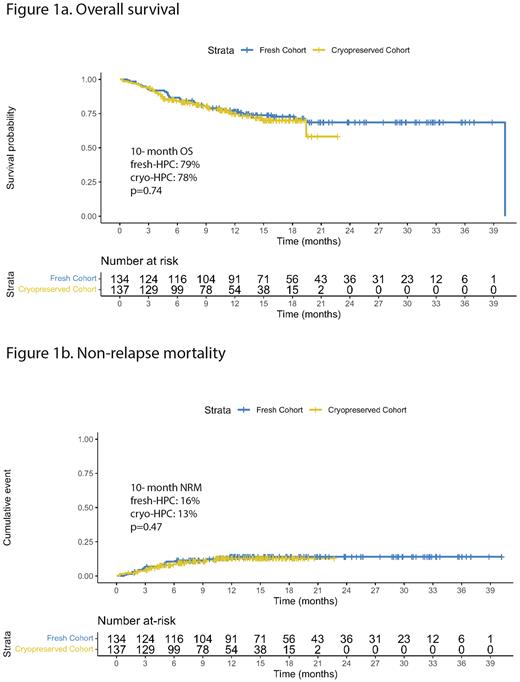
The median follow-up was 16 months in fresh [IQR 10, 25] vs 10 months in the cryo-HPC group [IQR 5, 15] (p<0.01). Median overall survival (OS) was not reached in either group, and the 10-month OS was similar in both groups (79% fresh-HPC vs 78% cryo-HPC, p=0.74). There was no difference in OS between the groups in the patients with a diagnosis of acute leukemia (p=0.57).
Relapse rates were similar (27% fresh-HPC vs 23% cryo-HPC, p=0.60) with a median time to relapse of 5 months [IQR 3, 12] vs 3 months [IQR 3, 7] respectively (p=0.07). 10-month non-relapse mortality was 16% in fresh-HPC vs 13% in the cryo-HPC group (Fig 1b, p=0.47). There were no significant differences in causes of death; disease relapse was the most common cause of death (51% fresh-HPC, 56% cryo-HPC).
Median neutrophil engraftment was faster in patients receiving fresh-HPC at 18 days [IQR 15, 22] vs 20 [IQR 17, 23] with cryo-HPC (p<0.01). Median platelet engraftment was also faster for fresh-HPC with 19 days [IQR 16, 23] compared with 22 [IQR 18, 29] with cryo-HPC (p<0.01). There was 1 case of primary GF in the fresh-HPC compared to 5 in the cryo-HPC groups (p=0.07), and 4 cases of secondary GF when using fresh-HPC compared to 3 with cryo-HPC (p=0.46).
The incidence of grade II-IV acute GVHD was 30% in fresh HPC and 23% in cryo-HPC (p=0.06). The preliminary incidence of chronic GVHD was 43% in fresh HPC vs 33% in cryo-HPC (p=0.01).
Conclusion Cryo-HPC may have prolonged time to neutrophil and platelet engraftment compared to fresh-HPC as well as increased incidence of primary graft failure. There were no significant differences in OS, relapse or acute GVHD among patients receiving fresh vs cryo-HPC in our cohort. Chronic GVHD was more common with fresh-HPC, likely due to longer follow-up times. These findings are consistent with those reported in literature.
Disclosures
Chung:Takeda: Honoraria; Astellas: Honoraria. Nevill:Abbvie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Alexion: Membership on an entity's Board of Directors or advisory committees; Taiho: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Sanford:Astellas: Other: Advisory Board ; Abbvie: Other: Advisory Board ; Pfizer: Research Funding. Song:Amgen: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria, Research Funding. Sutherland:Karyopharm: Research Funding; GSK: Research Funding; Janssen: Consultancy, Research Funding; Amgen: Consultancy; Celgene: Consultancy; Sanofi: Consultancy. Hay:Janssen: Research Funding; Kite/Gilead: Honoraria; BMS: Honoraria; Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal